X-ray Photoelectron Spectroscopy (XPS) is the most widely used characterization method in surface science. By using the photoelectric effect, which earned Albert Einstein the Nobel Prize in Physics in 1921, the XPS technique not only identifies elements present in a material but also reveals their chemical bonding environments.
In a Perspective article published in Nature Reviews Materials in November 2024, Professors Grzegorz Greczynski and Lars Hultman from the Department of Physics, Chemistry, and Biology (IFM) at Linköping University discuss critical insights into the XPS technique and emphasize the urgent need for new referencing standards in XPS.
In the 1950s, Kai Siegbahn and his research group at Uppsala University pioneered precise methods for analyzing the energy of electrons emitted from atoms, molecules, or radioactive materials when excited by X-rays. In 1954, they recorded the first high-energy-resolution XPS spectrum of cleaved sodium chloride and a few years later they reported the first observation of the chemical shift in copper oxide. Siegbahn’s groundbreaking work in transforming XPS into a robust analytical tool earned him the Nobel Prize in Physics in 1981.
XPS challenges
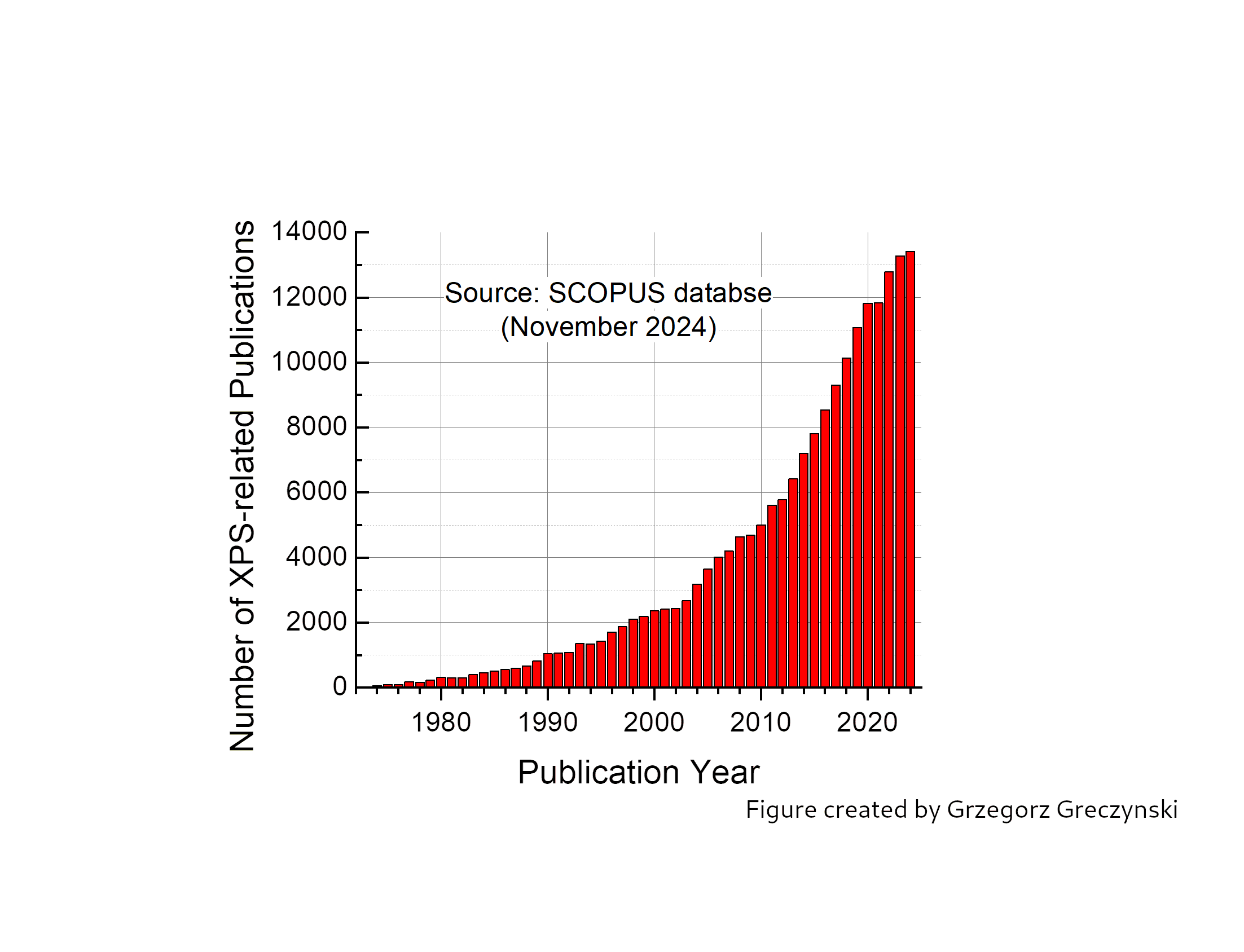
While XPS is straightforward to use for metallic systems, it poses profound challenges for insulating materials. In insulating materials, positive charge buildup during photoemission can cause uncontrolled shifts in binding energies of spectral peaks, leading to inaccurate spectra interpretation. Unlike metals, where the issue is essentially absent due to high electrical conductivity, no universal solution exists for insulators. This was already pointed out in the early days of XPS. The exponential growth in publications using XPS in recent years (see figure) has also increased the risk of potentially erroneous results being disseminated. This is a serious concern because scientific progress depends on reliable and reproducible data.
-Of course, in the pioneering days, more than fifty years ago, the resolution of XPS was much lower than that of today’s spectrometers, and therefore such errors were considered negligible. However, today, with instruments offering an order of magnitude better resolution than in the 60s, such errors can lead to misleading results and contribute to an unacceptably large spread in the binding energy values reported for the same chemical state, explains Lars Hultman, Professor at Linköping University and WISE researcher.
How can we solve these issues?
The persistence of referencing problems stems partly from a cultural tendency to follow established routines and equipment manufacturer instructions without revisiting foundational assumptions. Professor Hultman emphasizes the need for special attention and revision of energy referencing in XPS to protect the quality of produced science.
– In this Perspective, we review essential concepts and key experiments related to binding energy referencing. We discuss energy diagrams and appropriate reference levels for metallic and insulating samples with and without electrical contact with the sample holder. Using defined criteria for an ideal charge-reference method, we critically evaluate common referencing techniques, says Prof. Hultman.
To learn more about Professors Greczynski and Hultman see their latest publication, Binding energy referencing in X-ray photoelectron spectroscopy [Nat. Rev. Mater. (2024)]
To learn more about Prof. Hultman’s research in WISE read:
Eco-Deposition to Create Novel Multifunctional Coating Materials